|
Atomic Mass: Atomic mass of
an element is the number of protons + the number of
electrons.
Weight of 1Mol of an element
(in grams) is equal to the atomic mass.
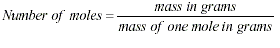
In an equation, the mass of the
reactants is equal to the mass of the products.
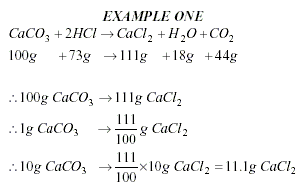
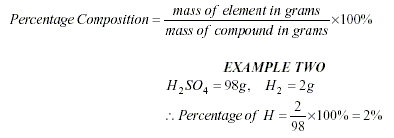
To calculate an Empirical
Formula
-
Write the percentages (or masses)
under the symbols.
-
Divide the percentages (or masses)
by the atomic mass.
-
Divide the smallest number to get a
simple ratio.
-
Write the numbers to the
bottom-right of each element, to get a formula.
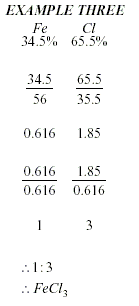
-
One mole of any gas occupies
24dm3(24l) at room temperature and
pressure.
-
Formulae are made up of
different elements expressed as symbols.
-
The bottom-right hand number is a
formula, is the number of atoms of that element.
Top
|